未選択
-
GLP-1 weight loss miracle drug semaglutide: new functions help improve the health status of AIDS patients
Recently, good news about the GLP-1 weight loss drug semaglutide (trade name: Wegovy) has come one after another. It is said that on March 7, 2023, Novo Nordisk revealed at its "Capital Markets Day" event that Wegovy is expected to be approved for listing in China this year. Subsequently, the US FDA announced on March 8 that it had approved Wegovy's new indication - to reduce the risk of cardiovascular death, heart attack and stroke in adults with cardiovascular disease and obesity or overweight.
Two studies reported last week at the Retrovirus and Opportunistic Infections Conference in Denver, Colorado, showed that the weight-loss drug semaglutide may help improve the health of HIV patients by reducing the number of infections associated with antibiotics. Retroviral treatment was associated with weight and fat accumulation and also reduced their chronic inflammatory response.
Among people living with HIV, the number of people who are overweight or obese is increasing, sparking interest in drugs like semaglutide among affected patients and doctors. However, so far, few studies have looked at the impact of these best-selling weight-loss drugs on HIV-infected people.
Semaglutide is a type of GLP-1 receptor agonist developed by Novo Nordisk. It controls appetite by simulating glucagon-like peptide 1, thereby achieving blood sugar lowering and weight loss. The drug is called Wegovy when used to treat obesity and Ozempic when used to treat diabetes.
Although the incidence of obesity in people with HIV is similar to trends in the general population, certain antiretroviral therapies used to suppress the HIV virus may contribute to weight gain and related morbidity in these patients. In addition, antiretroviral therapy is associated with abnormal fat accumulation, a metabolic-related fatty liver disease that affects approximately 30% to 40% of HIV-infected individuals. As the disease progresses, it can lead to liver failure and cardiovascular disease.
People with HIV are susceptible to more severe fatty liver disease, and there are currently no approved drugs to treat this condition.
Recent research data shows that among 222 HIV-infected patients treated with semaglutide, these patients lost an average of 6.5 kilograms in approximately one year, equivalent to 5.7% of their initial body weight.
At the Conference on Retroviruses and Opportunistic Infections, Jordan Lake of the University of Texas Health Science Center at Houston reported on the effectiveness of weekly injections of semaglutide for about six months in HIV patients with metabolic dysfunction-related fatty liver disease. The effectiveness of treatment in infected people. The study results showed that 29% of patients experienced complete remission of fatty liver disease. Jordan Lake noted that the study observed a significant reduction in patients' abnormal accumulation of liver fat, even over a short period of time.
The study also found that patients who received semaglutide experienced a decrease in muscle mass, with individuals aged 60 and older being most affected. Older HIV-infected individuals are more susceptible to semaglutide-related muscle loss and require close monitoring by their healthcare provider. It is worth noting that despite the success of GLP-1 weight loss drugs, more and more companies are beginning to focus on muscle-building therapies to combat the loss of muscle mass that may be caused by rapid weight loss.
In addition, there was a report at the Retrovirus and Opportunistic Infections Conference on the use of semaglutide in the treatment of lipohypertrophy in HIV patients. The disease is primarily characterized by abdominal fat accumulation, accompanied by increased inflammation and increased cardiometabolic risk. Current treatments for this disease are limited and ineffective.
In the report, Allison Eckard of the Medical University of South Carolina conducted a clinical trial in HIV-infected people with fatty liver disease. The results showed that semaglutide helped reduce abdominal fat accumulation in patients, and that patients who used semaglutide had nearly 40% lower levels of the inflammatory blood marker C-reactive protein than those who did not use it. This may have important positive consequences for people living with HIV, as even people living with HIV in good disease status may develop a chronic inflammatory state, and this increased inflammation may contribute to various end-organ diseases, including cardiovascular disease, and may Affects liver, kidneys, brain and cognitive function.
Comprehensive recent clinical studies show that semaglutide not only helps HIV-infected patients lose weight, but also reduces their fat accumulation and related chronic inflammation. This suggests that people living with HIV may be the latest group to benefit from GLP-1 weight-loss drugs. If the therapeutic efficacy of these early clinical studies is confirmed, GLP-1 weight loss drugs such as semaglutide may be key to controlling the metabolic problems often caused by HIV treatment.
Reference: https://www.nature.com/articles/d41586-024-00691-8
PR -
What are the advantages of peptide drugs and what are the current types of peptide drugs?
Peptides hold a unique position in the field of drug development, and since the emergence of therapeutic insulin in 1922, peptides have played an important role in medical practice. So far, more than one hundred polypeptide drugs have been approved for marketing in the world, and are widely used in the treatment of diabetes, tumor, chronic pain, multiple sclerosis and other diseases. Previously, we have introduced the origin and development of peptides and peptide drugs, and explained the classification and screening of peptide drugs (see previous tweets: Peptides – Unique Drugs).
Today, let’s have a hardcore science popularization on peptide drugs!
1.Peptides, what are their advantages?
Peptides are molecules composed of amino acids as the basic unit, with a molecular weight generally below 10 KDa, between small chemical molecules and biological products. Their main characteristics are high selectivity and low concentration of action. Classic therapeutic peptides such as hormones, growth factors, ion channel ligands, etc. trigger intracellular effects by binding to receptors.
Compared with biological agents such as antibodies and therapeutic proteins, peptides have a similar mode of action, but their immunogenicity is lower. Additionally, due to their ability to be chemically synthesized, their production cost is also lower.
Compared with small molecule drugs, peptides have a larger molecular weight and can more effectively inhibit protein-protein interactions (PPI), with higher selectivity and specificity, lower concentration of action, and lower side effects (Figure 1) [1] [2].
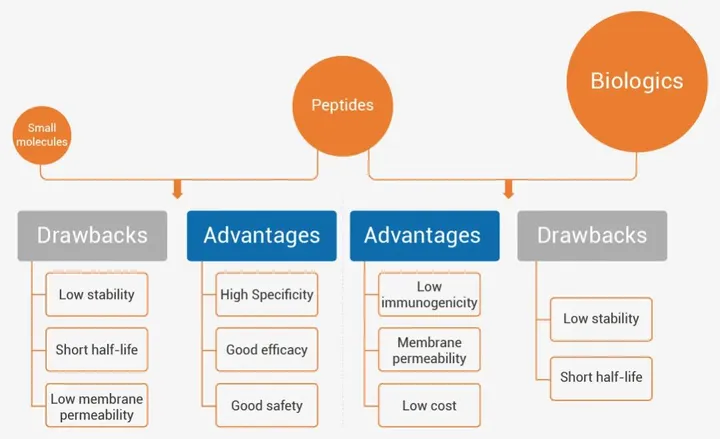
Figure 1 Comparison of advantages and disadvantages between peptides, small molecules, and biological agents [1] 2.What are peptide drugs available?
2.1 Hormonal peptides and their derivative drugs
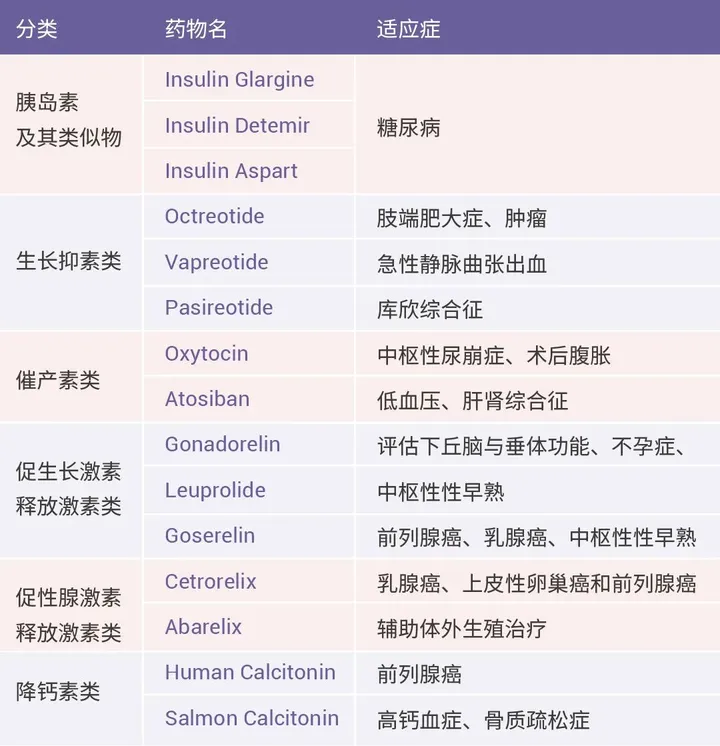
Generally speaking, there are four main medicinal peptides in clinical practice, one of which is hormone peptides and their derivatives. Due to the short half-life and high synthesis cost of peptides, early peptide drug development mainly focused on the field of low concentration human hormone peptides. The research on peptide drugs began with insulin, followed by the emergence of short peptide drugs such as oxytocin, antidiuretic hormone, somatostatin, and gonadotropin-releasing hormone, which opened up and enriched this field (Table 1). Many hormone drugs are still in use today.
With continuous scientific research, people have improved the characteristics of peptide hormones through chemical modifications such as C-terminal amidation, D-type amino acids, cyclization, and conjugation of long-chain fatty hydrocarbons.
For example, Octreotide and Pasireotide, which are based on somatostatin β Transforming the pharmacophore for modification successfully extended its half-life. In addition, drug development can also be based on the development of agonists or antagonists related to peptide hormones, such as Goserelin and Cetrorelix [3].
Table 1 Partial hormone peptides and their derivative drugs [3]
2.2 Natural Peptide Products
In addition to human derived peptides, there are also natural peptide products from plants, microorganisms, and other sources. Typical natural active peptides mainly include secondary metabolites of microorganisms and active peptides isolated from amphibian and insect venom. ICK peptides are a classic class of venom peptides, whose disulfide bond structure provides them with extraordinary stability and resistance to proteases, making them suitable as drug leads [3]. Ziconotide is an ICK peptide derived from toxic cone-shaped snails, which has good analgesic effects (Figure 2) [4].
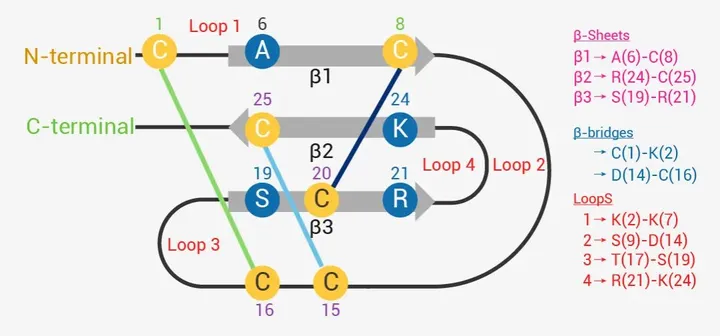
Figure 2 Structure of Ziconotide in Solution [4] 2.3 Peptide vaccines
Of course, there is also a peptide vaccine that must be mentioned. It is a subunit vaccine made from peptides, which can act as an immunogen to stimulate the body to produce an immune response by simulating the epitope sequence of antigens. Multimeric001, a representative type of anti infective peptide vaccine, contains epitopes of influenza virus B, T helper cells, and cytotoxic T cells, which can prevent various types of A and B influenza viruses. It has entered clinical stage III [5].
Compared with traditional inactivated and attenuated vaccines, polypeptide vaccines can not only be used as preventive vaccines against infectious or non infectious diseases, but also be used to treat Alzheimer’s disease, malignant tumors and other diseases [6]. Disomotide (G209-2M, IMDQVPFSV) is a melanoma antigen developed based on Gp100:209-217 (ITDQVPFSV), which can promote the production of cytotoxic T lymphocytes (CTL), recognize natural G209 and melanoma cells, and is currently in phase III clinical trials (Figure 3) [7] [8].
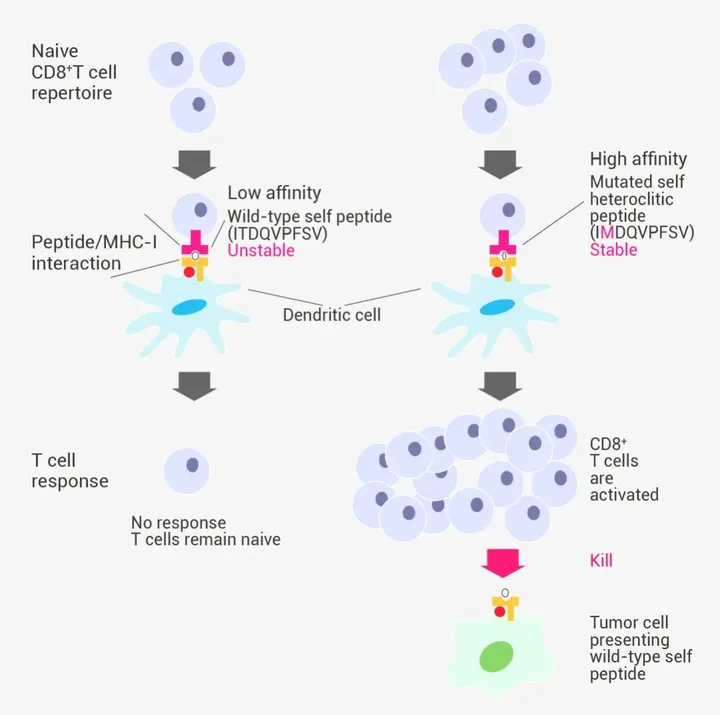
Figure 3 The mechanism of action of Diomotide [8]. The antigenic peptide IMDQVPFSV (Disaotide) that binds to the natural epitope ITDQVPFSV after point mutation exhibits stronger immunogenicity.
2.4 PDC peptide drugs
In addition, peptide drug delivery systems primarily based on PDC are also one of the directions of clinical research. Due to its excellent biological activity, non toxicity, and good compatibility, peptides can be used as drug carriers for peptide drug conjugates (PDC). At present, 177 Lu dotata (lutathera) ®) It has been approved by the FDA for the treatment of neuroendocrine tumors, and there are still many PDCs currently in the clinical or preclinical stage. PDC is composed of peptides covalently bound to drugs through ligands, retaining the peptide’s function and biological activity. At the same time, it also utilizes the cleavability of the ligands to release drugs in a responsive manner, thereby improving drug circulation stability and targeting in vivo, and reducing drug toxicity and side effects (Figure 4) [9].
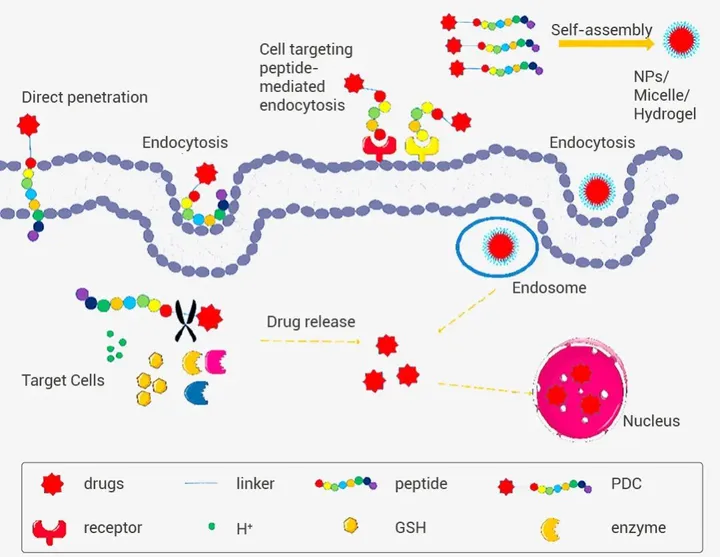
Figure 4 PDC directly penetrates or internalizes into cells through peptides, releasing drugs [9] 3.Summary
This issue introduces the characteristics of peptide drugs and four main types of medicinal peptides, and combines specific cases to further deepen our understanding of peptide drugs. At present, peptide drugs have been in clinical practice for a century, and classic hormone drugs still dominate the main market. The two major bottlenecks of inconvenient delivery methods and frequent delivery cycles still need to be solved urgently. The constantly emerging new technologies such as PDC and multifunctional peptides also need to be explored and enriched by friends~
The advantage of the KS-V Peptide integration service platform is to launch a catalog of peptide products with scientific research value. Each peptide product is purified by HPLC, resulting in more stable quality and timely delivery. The application scenarios cover new hotspots and valuable research fields, such as protein purification and detection, disease-related research, immunology and biochemistry research, scientific research peptides, medicinal peptides, etc., to meet the needs of researchers at different stages. We have a complete customer service system and technical team, enjoying a one-on-one specialized service experience, and providing you with professional services in a timely manner.
-
If my peptide is 95% pure, what is the other 5%?
In peptide synthesis, when a peptide is reported as being 95% pure, it means that 95% of the material in the sample consists of the desired peptide sequence. The remaining 5% is composed of impurities or related substances. These impurities can arise from various sources during the synthesis, purification, and handling processes. Common impurities in peptide synthesis include:
-
Incomplete Coupling Products: These are peptides where not all amino acids have successfully coupled together during the synthesis, leading to shorter-than-desired sequences.
-
Deletion Sequences: These are peptides where one or more amino acids are missing in the sequence due to incomplete reactions during the synthesis.
-
Side-Chain Protecting Group Adducts: The protecting groups used during the synthesis may not be completely removed, leading to adducts with the peptide.
-
By-Products of Coupling Reactions: Some reactions may produce unwanted by-products that can contribute to impurities in the final product.
-
Residual Reagents: Traces of reagents used in the synthesis or purification steps may remain in the final product.
Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) are commonly employed to assess the purity of synthesized peptides and identify the nature of impurities present. These techniques allow researchers to characterize the composition of the peptide sample and make adjustments to the synthesis or purification process if necessary.
Website: https://www.ks-vpeptide.com/aboutus.html -
-
How are peptides purified?
Peptide purification is a crucial step in the production of high-quality peptides for various applications, such as research, pharmaceuticals, and diagnostics. The most common method for peptide purification is high-performance liquid chromatography (HPLC), specifically reverse-phase HPLC (RP-HPLC). Here's an overview of the peptide purification process:
-
Solid-Phase Peptide Synthesis (SPPS):
- Peptide synthesis often begins with solid-phase peptide synthesis. This method involves the stepwise addition of protected amino acids to a solid support, building the peptide chain in the C-to-N direction.
- After synthesis, the peptide is still attached to the solid support.
-
Cleavage and Deprotection:
- The peptide is cleaved from the solid support, and any remaining protecting groups are removed. This step often involves treatment with a cleavage cocktail, which may include acids or other reagents.
-
Crude Peptide:
- The resulting mixture, known as the crude peptide, contains the desired peptide along with any incomplete sequences, deletion sequences, or side products from the synthesis.
-
Purification by RP-HPLC:
- RP-HPLC is the most widely used technique for peptide purification. In this method, a column with a hydrophobic stationary phase is used, and peptides are eluted based on their hydrophobicity.
- A gradient of increasing organic solvent (typically acetonitrile) in an aqueous buffer is applied to separate peptides based on their hydrophobic interactions with the column.
- The fractions containing the purified peptide are collected, and the purity is typically assessed using analytical HPLC and other techniques.
-
Analysis and Characterization:
- The purity and identity of the purified peptide are verified using analytical techniques such as mass spectrometry and analytical HPLC. Mass spectrometry provides accurate mass information, while analytical HPLC assesses the purity of the sample.
-
Optional Additional Steps:
- Depending on the application, additional purification steps or modifications may be required, such as further HPLC steps, ion-exchange chromatography, or size-exclusion chromatography.
-
Lyophilization:
- The purified peptide is often lyophilized (freeze-dried) to obtain a stable powder form that can be easily stored and reconstituted.
It's important to note that the purification strategy may vary based on the characteristics of the peptide, the scale of synthesis, and the intended application. Additionally, automated peptide synthesizers and purification systems are commonly used to streamline and optimize the process.
Website: https://www.ks-vpeptide.com/aboutus.html -
-
2023 global peptide event overview delivery
Part1 Financing/BD Event
December 2023
- Kintide Pharmaceuticals completed US$8 million in seed round financing to advance a highly integrated computational design-automated synthetic peptide drug discovery platform
- Roche acquires Carmot Therapeutics for over US$3 billion, entering the GLP-1 competition
- Fractyl Health said in a regulatory filing that it plans to conduct an IPO in 2024. The company is committed to developing innovative treatments for type 2 diabetes and obesity, and the funds raised will be used to advance the development of its pipeline, including a gene therapy that expresses a GLP-1 receptor agonist in the pancreas.
- Amide Technologies announced that it has begun commercializing its novel peptide manufacturing platform and has completed
$16.5Mnin financing, including a$7.5MnSeries A+ round led by Engine Ventures with participation from Forcefield Venture Fund. It previously raised a $3.55Mn Series A round led by Engine Ventures and a $5.45Mn Seed round led by Biological Engineering Ventures. - Alkermes plc announced that it has entered into a definitive agreement to sell its development and manufacturing facility in Athlone, Ireland, to Novo Nordisk. Under the terms of the agreement, upon completion of the transaction, Alkermes will be entitled to a one-time cash payment of
$92.5Mnfor the facility and associated assets, subject to customary adjustments in accordance with the agreement.
November 2023
- Zhifei Biotech plans to acquire 100% equity of Chongqing Chenan Biopharmaceutical Co., Ltd. in cash to enter the GLP-1 field
- Chengdu Taihe Weiye Biotechnology Co., Ltd. completed nearly 300 million yuan in Series A+ financing to accelerate the company’s Fmoc amino acid production capacity construction
- Peptide Biotech completed nearly 200 million yuan in Series B+ financing to promote the development of GLP-1 products
- lmagine Pharma completed US$32.5 million in Series A financing to develop new peptide IMG-1 and other projects
September 2023
- PeptiDream enters into a new multi-target collaboration and licensing agreement with Genentech, a Roche company, to discover and develop novel macrocyclic peptide-radioisotope (peptide-RI) conjugates
- Elicio Therapeutics receives $2.6 million foundation grant to fund research into two therapeutic cancer peptide vaccines
- Shenzhen Tuosheng Biotechnology Co., Ltd. announced the completion of tens of millions of RMB Pre-A round of financing
August 2023
- PeptiGrowth Inc (PeptiGrowth) and Orizuru Therapeutics, Inc (OZTx) have signed a joint development agreement to create a new synthetic peptide to replace recombinant growth factors used in the production of regenerative medicine products
- HBC lmmunology completed US$900,000 in seed round financing to advance the research and development of peptide drugs
June 2023
- Sino Biopharmaceuticals and Hongyun Huaning (Hangzhou) Biopharmaceutical Co., Ltd. have reached a cooperation agreement to jointly develop the dual-target innovative weight loss drug GMA106. GMA106 is a dual-target drug mainly suitable for the treatment of obesity, non-alcoholic fatty liver disease and diabetes
May 2023
- Carmot Therapeutics announces raising $150 million in super Series E funding to advance its development of treatments for obesity and diabetes
- lronwood successfully acquires next-generation GLP-2 through acquisition of VectivBio for $1 billion
Annual Highlights of Blockbuster Products
December 2023
- Eli Lilly announced that its injectable Zepbound (Tirzepatide) is now available in U.S. pharmacies
- Lisata Therapeutics announced that it has completed enrollment in the Phase IIb ASCEND study of its novel drug LSTA1 for the treatment of metastatic pancreatic ductal adenocarcinoma (“mPDAC”), achieving a key milestone.
- Italian company Nerviano Medical Sciences (NMS) and Spanish pharmaceutical company Italfarmaco (ITF) jointly announced that ITF will use NMS’s proprietary linker-payload technology to develop a new PDC product candidate.
- Apellis presented post-hoc data for its bicyclic peptide drug EMPAVELI® (pegcetacoplan) at the ASH Annual Meeting that strengthen the efficacy of EMPAVELI (pegcetacoplan) in adults with paroxysmal nocturnal hemoglobinuria (PNH) for up to three years. years of long-term efficacy and safety.
November 2023
- Eli Lilly announced that its GIP/GLP-1 receptor dual agonist Zepbound (Tirzepatide) has been approved by the U.S. FDA to reduce weight and maintain weight stability in obese or overweight adult patients.
- Novo Nordisk released its results for Q3 and the first three quarters of 2023. Sales of semaglutide in the first three quarters have exceeded US$14.2 billion.
- Eli Lilly and Company released its financial report for the third quarter of 2023. Sales of Tirzepatide in the first three quarters have reached US$2.957 billion.
October 2023
- Novo Nordisk announced that the Phase III clinical study FLOW of semaglutide in patients with type 2 diabetes and chronic kidney disease with renal insufficiency was terminated early due to excellent efficacy.
August 2023
- Eli Lilly’s Tirzepatide Injection Submits Registration Application in China for Long-term Weight Management Indication in Adults
July 2023
- Eli Lilly announced the results of two phase 3 clinical trials on tilpotide. The results showed that the average weight loss effect was as high as 26.6%, making it the most powerful weight loss drug in history.
Peptide drugs approved for marketing
December 2023
- Tonghua Dongbao Pharmaceutical’s marketing application for biosimilar GLP-1 analog liraglutide injection filed under registration classification 3.3 has been approved
- Ascendis Pharma announced that the FDA has accepted the company’s resubmitted New Drug Application for palopegteriparatide (TransCon PTH) for the treatment of adult patients with hypoparathyroidism.
- The National Medical Insurance Administration officially announced the adjustment list of the 2023 version of the National Medical Insurance Drug Catalog. The new round of medical insurance catalog includes four popular GLP-1 products. These include Renhui Biotech’s benaglutide injection, Lilly’s dulaglutide injection, Hanson Pharmaceuticals’ polyethylene glycol loxenatide injection, Novo Nordisk’s semaglutide injection, etc.
September 2023
- BioLineRx announces FDA approval of Aphexda (motixafortide) combined with filgrastim (granulocyte colony-stimulating factor G-CSF) to mobilize hematopoietic stem cells into the peripheral blood as a stem cell mobilization (SCM) to promote autologous transplantation in patients with multiple myeloma at the time of transplantation
- The marketing application for new indications of Lilly’s dulaglutide injection (trade name: Duida) was approved
July 2023
- Renhui Biotech’s benaglutide has been approved for marketing as a new indication for weight loss. This is the first original new weight loss drug in China, and the injection frequency is three times a day.
- East China Medicine’s Liraglutide Injection is approved for marketing for obesity or overweight indications
June 2023
- Hansoh Pharmaceutical’s Class 1 new drug “Pemoxatide Injection” (trade name: Saint Relais) was approved for marketing. This medicine is suitable for adult non-dialysis patients who are not receiving erythropoiesis stimulating agents (ESA), and adult dialysis patients who are receiving short-acting erythropoietin (EPO).
May 2023
- Blue Earth Diagnostics’ radiohybrid prostate-specific membrane antigen (PSMA)-targeted PET imaging agent Posluma (flotufolastat F 18) injection (formerly known as 18F-rhPSMA-7.3) was approved by the FDA for marketing
March 2023
- Acadia Pharmaceuticals Announces FDA Approval of DAYBUE (trofinetide) for the Treatment of Rett Syndrome in Adult and Pediatric Patients Two Years and Older
- Cidara Therapeutics’ New Drug Application (NDA) for Rezafungin for the treatment of candidemia and invasive candidiasis was approved by the FDA
January 2023
- Radius Health’s TYMLOS (abapatide) received U.S. FDA approval for new indication (osteoporosis)
Website: https://www.ks-vpeptide.com/
