未選択
-
NMR Analysis: A Powerful Tool for Peptide Characterization
Introduction to NMR Analysis
Nuclear Magnetic Resonance (NMR) analysis is a sophisticated analytical technique used to determine the structure, purity, and dynamics of molecules, including peptides and proteins. At KS-V Peptide, we utilize advanced NMR spectroscopy to ensure the highest quality and accuracy in peptide characterization.
Why NMR Analysis is Essential for Peptides
NMR spectroscopy provides detailed insights into:
- Molecular Structure: Confirms the 3D conformation of peptides.
- Purity Assessment: Detects impurities and confirms sample integrity.
- Dynamic Behavior: Analyzes molecular interactions and folding mechanisms.
Our NMR analysis services at KS-V Peptide ensure reliable and precise results for research and pharmaceutical applications.
Applications of NMR in Peptide Research
NMR is widely used in:
✔ Drug Development: Validates peptide-based drug structures.
✔ Biomolecular Studies: Examines protein-peptide interactions.
✔ Quality Control: Ensures batch consistency in peptide synthesis.Choose KS-V Peptide for Reliable NMR Analysis
With cutting-edge NMR technology and expert analysis, we deliver accurate and reproducible data for your peptide research needs.
Learn more about our NMR services: https://www.ks-vpeptide.com/nmr-analysis.html
Connect us with linkedin:https://www.linkedin.com/company/ks-v-peptide/PR -
Optimizing ADC Therapeutics: The Critical Role of Peptide Linkers in Targeted Drug Delivery
Introduction
Antibody-Drug Conjugates (ADCs) represent a groundbreaking advancement in targeted cancer therapy, and peptide linkers play a pivotal role in their stability, efficacy, and safety. This article explores the importance of peptide linkers in ADC design and highlights the expertise of KS-V Peptide in developing high-performance linker solutions.
Why Are Peptide Linkers Essential for ADCs?
-
Controlled Payload Release
Peptide linkers enable selective drug release in the tumor microenvironment through enzymatic cleavage or pH-sensitive mechanisms, minimizing off-target toxicity. -
Enhanced Stability
Optimized peptide linkers improve ADC stability in systemic circulation, preventing premature payload release and ensuring efficient drug delivery. -
Customizable Design
Linker chemistry can be tailored to match specific antibodies, payloads, and therapeutic requirements, allowing for precision-engineered ADCs.
KS-V Peptide’s Expertise in ADC Linker Development
As a trusted innovator in peptide technology, KS-V Peptide offers:
-
Protease-cleavable & non-cleavable linkers for diverse ADC platforms
-
Site-specific conjugation technologies for homogeneous ADC production
-
GMP-grade manufacturing with strict quality control
The Future of ADC Optimization
Next-generation peptide linkers are driving advancements in:
✔ Improved therapeutic windows (higher efficacy, lower toxicity)
✔ Novel conjugation strategies for better DAR (Drug-to-Antibody Ratio) control
✔ Dual-payload ADCs enabled by smart linker systemsPartner with ADC Linker Experts
Elevate your ADC development with KS-V Peptide’s cutting-edge peptide linker solutions.
Learn more: https://www.ks-vpeptide.com/peptide-linkers-for-adc
Connect on LinkedIn: https://www.linkedin.com/company/ks-v-peptide/ -
-
What Is NMR Analysis and Why It Matters in Modern Molecular Research
Nuclear Magnetic Resonance (NMR) analysis is one of the most powerful and reliable techniques used for identifying the structure, purity, and dynamics of molecules. It plays a vital role in a wide range of scientific disciplines including organic chemistry, biochemistry, pharmaceuticals, and peptide drug development.
At KS-V Peptide, we utilize advanced NMR technology to support accurate structural elucidation and quality control of custom peptides and small molecules. Learn more about our NMR Analysis services.
What Is NMR Analysis?
NMR analysis is a non-destructive analytical method that detects the magnetic properties of certain atomic nuclei. When exposed to a strong magnetic field and radiofrequency pulse, these nuclei resonate at a frequency specific to their chemical environment. The resulting NMR spectrum provides detailed information about the molecular structure, functional groups, and chemical environment.
Applications of NMR Analysis
NMR spectroscopy is widely used in:
-
Peptide structure verification
-
Pharmaceutical compound development
-
Metabolomics and biomarker discovery
-
Material science and polymer analysis
-
Natural product identification
Especially in peptide research, NMR helps confirm the sequence, folding, and purity of synthetic peptides, ensuring that researchers and developers work with precise molecular data.
Advantages of NMR Analysis
-
✅ Non-destructive: Samples can often be recovered after analysis
-
✅ Quantitative and qualitative: Provides both structure and concentration details
-
✅ Highly accurate: Delivers molecular-level insights with high resolution
-
✅ No need for derivatization: Unlike some other techniques, NMR often works directly on the original compound
Why Choose KS-V Peptide for NMR Analysis?
At KS-V Peptide, we specialize in custom peptide development and characterization. Our team of scientists leverages NMR to ensure structural confirmation and quality assurance for every peptide delivered. We support research institutions and pharmaceutical companies with fast, reliable, and professional analysis services.
Explore our NMR Analysis service to see how we can support your drug discovery and development workflow.
Stay Connected
Want to stay updated on the latest in peptide technologies and analytical methods?
Follow KS-V Peptide on LinkedIn for insights, case studies, and industry news. -
-
Phage Display: A Powerful Tool for Protein Engineering and Drug Discovery
Abstract: Phage display is a versatile and powerful technique used in protein engineering and drug discovery. It involves the presentation of peptide or protein libraries on the surface of bacteriophages, allowing for the selection of specific binding molecules through affinity selection. This article provides an overview of the phage display technique, its applications in various fields, and recent advancements in the field.
Introduction: Phage display has revolutionized the field of molecular biology by enabling the rapid screening and selection of peptides and proteins with desired properties. Originally developed by George Smith in 1985, this technique has since been widely adopted in diverse areas such as antibody engineering, vaccine development, and targeted drug delivery. The ability to generate vast libraries of randomized peptides or proteins and select for molecules with high affinity and specificity has made phage display an indispensable tool in modern biotechnology and pharmaceutical research.
Methodology: Phage display relies on the construction of combinatorial libraries of peptides or proteins fused to the coat proteins of filamentous bacteriophages, such as M13 or fd. These libraries typically consist of billions of unique variants, providing a vast pool of potential binding molecules. Selection of specific binders is achieved through multiple rounds of affinity screening, wherein the phage library is exposed to a target molecule (e.g., a protein, cell, or small molecule), non-binding phages are washed away, and bound phages are eluted and amplified for subsequent rounds of selection. Through iterative cycles of selection and amplification, highly specific binders can be isolated and characterized.
Applications: Phage display has numerous applications in both basic research and applied biotechnology. In antibody engineering, phage display has been instrumental in the generation of monoclonal antibodies with therapeutic potential, as well as the discovery of antibody fragments with improved properties such as increased affinity or stability. In drug discovery, phage display libraries can be screened against targets of interest to identify novel peptide or protein ligands with potential therapeutic applications. Additionally, phage display has been utilized in vaccine development, epitope mapping, protein-protein interaction studies, and diagnostics.
Recent Advancements: Recent advancements in phage display technology have further expanded its capabilities and applications. For instance, the development of synthetic biology tools has enabled the construction of more complex and diverse phage libraries, allowing for the screening of larger and more diverse sequence spaces. Moreover, advances in high-throughput sequencing and bioinformatics have facilitated the rapid analysis and characterization of phage display libraries, accelerating the identification of lead candidates for various applications. Additionally, efforts to engineer phage display systems with enhanced stability, specificity, and functionality are ongoing, further enhancing the utility of this technique in biotechnology and medicine.
Conclusion: Phage display is a versatile and powerful technique that continues to drive innovation in protein engineering and drug discovery. Its ability to generate and screen vast libraries of peptides and proteins makes it an invaluable tool for identifying novel binders with therapeutic potential. As technology advances and our understanding of phage biology deepens, phage display is poised to remain at the forefront of biomedical research, offering new opportunities for the development of diagnostics, therapeutics, and biotechnological applications.
-
Peptide linkers for Antibody-drug conjugates (ADCs)
Antibody-drug conjugates (ADCs) are a type of targeted cancer therapy that combines the specificity of monoclonal antibodies with the cytotoxic effects of drugs. Peptide linkers play a crucial role in ADC design, as they connect the antibody and the cytotoxic payload, facilitating controlled release of the drug within the target cells. The choice of linker can impact stability, drug release kinetics, and overall efficacy of the ADC. The choice of a specific linker depends on factors such as the pharmacokinetics of the ADC, the desired release mechanism, and the characteristics of the drug payload. It's crucial to balance stability in circulation with efficient drug release at the target site to maximize the therapeutic effect of the ADC. Additionally, advancements in linker technology continue to contribute to the development of novel and improved ADCs for cancer therapy.
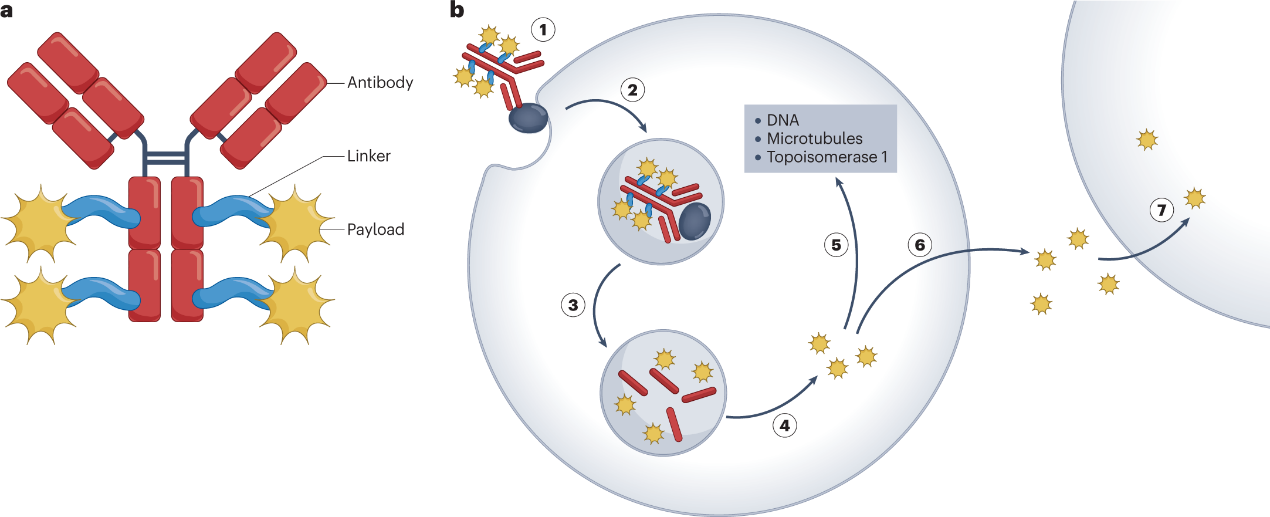
The advantage Of Peptide Linker
1. Biological Compatibility
Peptide linkers are composed of natural amino acids, which are biocompatible and less likely to induce an immune response. This can contribute to the overall safety profile of the ADC.
2. Specificity and Selectivity
Peptide linkers can be designed to incorporate specific cleavage sites for proteases that are overexpressed in the target cells. This allows for selective drug release within the tumor microenvironment, enhancing the therapeutic window.
3. Stability in Circulation
Peptide linkers can be engineered for stability in the bloodstream, minimizing premature drug release during circulation. This stability is crucial for maintaining the integrity of the ADC and preventing off-target effects.
4. Tunable Pharmacokinetics
The properties of peptide linkers, such as their size and hydrophilicity, can be fine-tuned to influence the pharmacokinetics of the ADC. This tunability allows for optimization of drug delivery and distribution in vivo.
5. Ease of Synthesis
Peptide synthesis techniques are well-established, making it relatively straightforward to design and produce peptide linkers. This ease of synthesis contributes to the scalability and cost-effectiveness of ADC manufacturing.
6. pH Sensitivity
Some peptide linkers can be designed to be pH-sensitive, allowing for drug release in the acidic environment of endosomes or lysosomes within target cells. This pH responsiveness enhances the specificity of drug delivery to cancer cells.
7. Multifunctionality
Peptide linkers can be engineered to have multiple functions, such as facilitating site-specific conjugation, improving solubility, or enhancing overall stability. This versatility contributes to the design of ADCs with optimized properties.
8. Well-Characterized Cleavage Mechanisms:
Proteolytic cleavage of peptide linkers by cellular proteases is a well-characterized biological process. This predictability allows for a better understanding of the drug release mechanism and facilitates rational design of ADCs.
Peptide linkers Table:
Product Name Structure M.W. Purity Fmoc-val-cit-PAB-OH 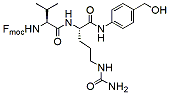
601.7 96% Boc-Val-Cit-PAB 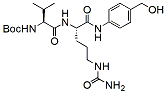
644.7 98% MC -Val-Cit 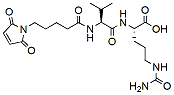
453.5 98% MC-Val-Cit-PAB-Gly 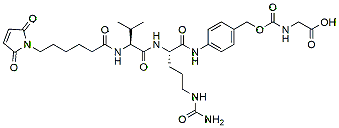
673.7 95% Alkyne-Val-Cit-PAB-OH 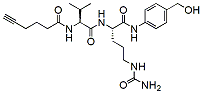
473.6 98% SPDP-Val-Cit-PAB-OH 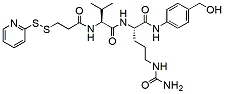
576.7 98% Fmoc-PEG2-Val-Cit-PAB-OH 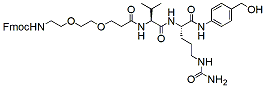
760.9 95% NH2-PEG3-Val-Cit-PAB-OH 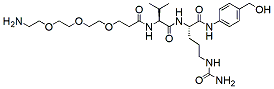
82.7 95% Mal-PEG2-Val-Cit-PAB-OH 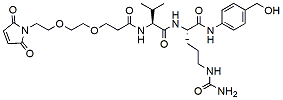
618.7 95% Mal-Amide-PEG4-Val-Cit-PAB-OH 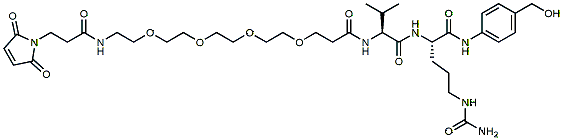
777.9 95% Azido-PEG3-Val-Cit-PAB-OH 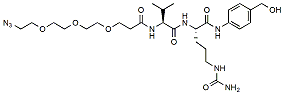
608.7 96% Azido-PEG4-Val-Ala-PAB 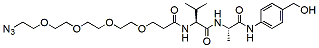
566.7 95% MC-Val-Ala-OH 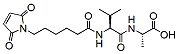
381.4 95% Mal-PEG4-Val-Ala-PAB 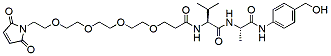
620.7 95% Boc-PEG4-Val-Ala-PAB 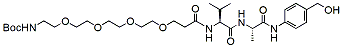
640.8 95% Fmoc-PEG4-Val-Ala-PAB 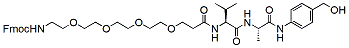
762.9 95% Mal-amido-PEG8-val-gly-PAB-OH 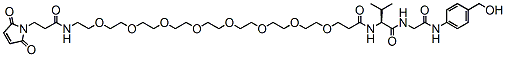
854.0 95%/td> Fmoc-Gly-Gly-Phe-Gly-OH 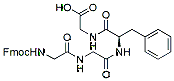
558.6 95% Gly-Gly-Phe-Gly 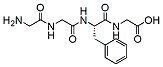
336.4 95% Mal-Gly-Gly-L-Phe-N-[(carboxymethoxy)methyl]Glycinamide 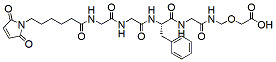
616.6 95% Mal-PEG8-Gly-Gly-L-Phe-N-[(carboxymethoxy)methyl]Glycinamide 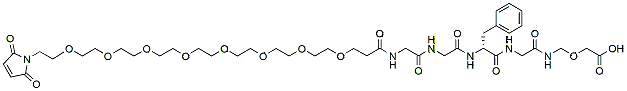
927.0 95% MC-Gly-Gly-Phe-Gly 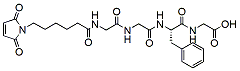
529.6 98% NH2-Glu-Gly-Cit-PAB-OH 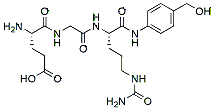
466.5 95% Fmoc-PEG4-Glu-Gly-Cit-PAB-OH 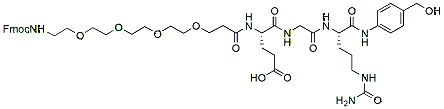
935.43 95% NH2-Sar10-COOH 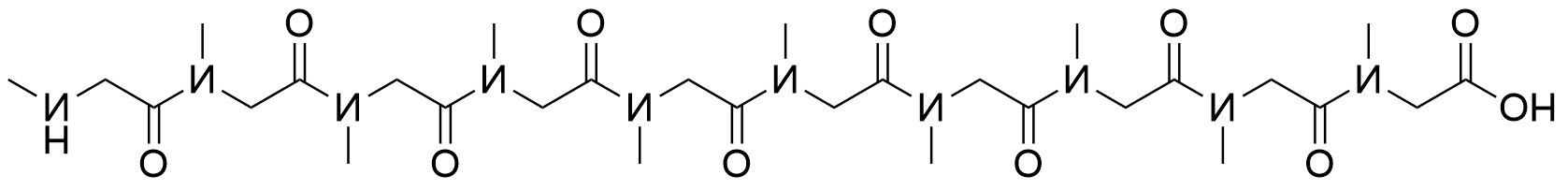
728.81 98% Fmoc-NH2-Sar10-COOH 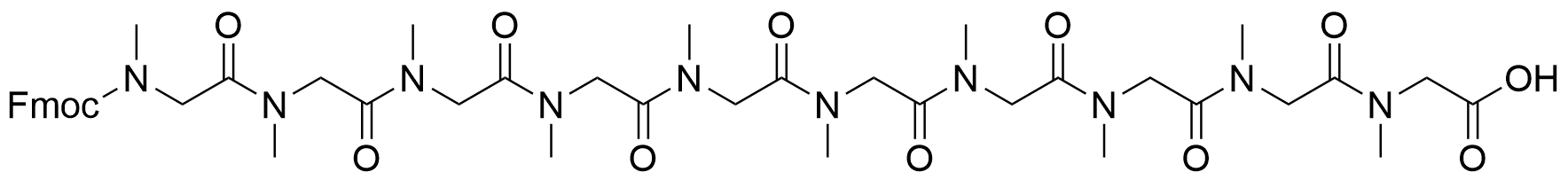
951.05 98%
