-
If my peptide is 95% pure, what is the other 5%?
In peptide synthesis, when a peptide is reported as being 95% pure, it means that 95% of the material in the sample consists of the desired peptide sequence. The remaining 5% is composed of impurities or related substances. These impurities can arise from various sources during the synthesis, purification, and handling processes. Common impurities in peptide synthesis include:
-
Incomplete Coupling Products: These are peptides where not all amino acids have successfully coupled together during the synthesis, leading to shorter-than-desired sequences.
-
Deletion Sequences: These are peptides where one or more amino acids are missing in the sequence due to incomplete reactions during the synthesis.
-
Side-Chain Protecting Group Adducts: The protecting groups used during the synthesis may not be completely removed, leading to adducts with the peptide.
-
By-Products of Coupling Reactions: Some reactions may produce unwanted by-products that can contribute to impurities in the final product.
-
Residual Reagents: Traces of reagents used in the synthesis or purification steps may remain in the final product.
Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) are commonly employed to assess the purity of synthesized peptides and identify the nature of impurities present. These techniques allow researchers to characterize the composition of the peptide sample and make adjustments to the synthesis or purification process if necessary.
Website: https://www.ks-vpeptide.com/aboutus.htmlPR -
-
How are peptides purified?
Peptide purification is a crucial step in the production of high-quality peptides for various applications, such as research, pharmaceuticals, and diagnostics. The most common method for peptide purification is high-performance liquid chromatography (HPLC), specifically reverse-phase HPLC (RP-HPLC). Here's an overview of the peptide purification process:
-
Solid-Phase Peptide Synthesis (SPPS):
- Peptide synthesis often begins with solid-phase peptide synthesis. This method involves the stepwise addition of protected amino acids to a solid support, building the peptide chain in the C-to-N direction.
- After synthesis, the peptide is still attached to the solid support.
-
Cleavage and Deprotection:
- The peptide is cleaved from the solid support, and any remaining protecting groups are removed. This step often involves treatment with a cleavage cocktail, which may include acids or other reagents.
-
Crude Peptide:
- The resulting mixture, known as the crude peptide, contains the desired peptide along with any incomplete sequences, deletion sequences, or side products from the synthesis.
-
Purification by RP-HPLC:
- RP-HPLC is the most widely used technique for peptide purification. In this method, a column with a hydrophobic stationary phase is used, and peptides are eluted based on their hydrophobicity.
- A gradient of increasing organic solvent (typically acetonitrile) in an aqueous buffer is applied to separate peptides based on their hydrophobic interactions with the column.
- The fractions containing the purified peptide are collected, and the purity is typically assessed using analytical HPLC and other techniques.
-
Analysis and Characterization:
- The purity and identity of the purified peptide are verified using analytical techniques such as mass spectrometry and analytical HPLC. Mass spectrometry provides accurate mass information, while analytical HPLC assesses the purity of the sample.
-
Optional Additional Steps:
- Depending on the application, additional purification steps or modifications may be required, such as further HPLC steps, ion-exchange chromatography, or size-exclusion chromatography.
-
Lyophilization:
- The purified peptide is often lyophilized (freeze-dried) to obtain a stable powder form that can be easily stored and reconstituted.
It's important to note that the purification strategy may vary based on the characteristics of the peptide, the scale of synthesis, and the intended application. Additionally, automated peptide synthesizers and purification systems are commonly used to streamline and optimize the process.
Website: https://www.ks-vpeptide.com/aboutus.html -
-
2023 global peptide event overview delivery
Part1 Financing/BD Event
December 2023
- Kintide Pharmaceuticals completed US$8 million in seed round financing to advance a highly integrated computational design-automated synthetic peptide drug discovery platform
- Roche acquires Carmot Therapeutics for over US$3 billion, entering the GLP-1 competition
- Fractyl Health said in a regulatory filing that it plans to conduct an IPO in 2024. The company is committed to developing innovative treatments for type 2 diabetes and obesity, and the funds raised will be used to advance the development of its pipeline, including a gene therapy that expresses a GLP-1 receptor agonist in the pancreas.
- Amide Technologies announced that it has begun commercializing its novel peptide manufacturing platform and has completed
$16.5Mnin financing, including a$7.5MnSeries A+ round led by Engine Ventures with participation from Forcefield Venture Fund. It previously raised a $3.55Mn Series A round led by Engine Ventures and a $5.45Mn Seed round led by Biological Engineering Ventures. - Alkermes plc announced that it has entered into a definitive agreement to sell its development and manufacturing facility in Athlone, Ireland, to Novo Nordisk. Under the terms of the agreement, upon completion of the transaction, Alkermes will be entitled to a one-time cash payment of
$92.5Mnfor the facility and associated assets, subject to customary adjustments in accordance with the agreement.
November 2023
- Zhifei Biotech plans to acquire 100% equity of Chongqing Chenan Biopharmaceutical Co., Ltd. in cash to enter the GLP-1 field
- Chengdu Taihe Weiye Biotechnology Co., Ltd. completed nearly 300 million yuan in Series A+ financing to accelerate the company’s Fmoc amino acid production capacity construction
- Peptide Biotech completed nearly 200 million yuan in Series B+ financing to promote the development of GLP-1 products
- lmagine Pharma completed US$32.5 million in Series A financing to develop new peptide IMG-1 and other projects
September 2023
- PeptiDream enters into a new multi-target collaboration and licensing agreement with Genentech, a Roche company, to discover and develop novel macrocyclic peptide-radioisotope (peptide-RI) conjugates
- Elicio Therapeutics receives $2.6 million foundation grant to fund research into two therapeutic cancer peptide vaccines
- Shenzhen Tuosheng Biotechnology Co., Ltd. announced the completion of tens of millions of RMB Pre-A round of financing
August 2023
- PeptiGrowth Inc (PeptiGrowth) and Orizuru Therapeutics, Inc (OZTx) have signed a joint development agreement to create a new synthetic peptide to replace recombinant growth factors used in the production of regenerative medicine products
- HBC lmmunology completed US$900,000 in seed round financing to advance the research and development of peptide drugs
June 2023
- Sino Biopharmaceuticals and Hongyun Huaning (Hangzhou) Biopharmaceutical Co., Ltd. have reached a cooperation agreement to jointly develop the dual-target innovative weight loss drug GMA106. GMA106 is a dual-target drug mainly suitable for the treatment of obesity, non-alcoholic fatty liver disease and diabetes
May 2023
- Carmot Therapeutics announces raising $150 million in super Series E funding to advance its development of treatments for obesity and diabetes
- lronwood successfully acquires next-generation GLP-2 through acquisition of VectivBio for $1 billion
Annual Highlights of Blockbuster Products
December 2023
- Eli Lilly announced that its injectable Zepbound (Tirzepatide) is now available in U.S. pharmacies
- Lisata Therapeutics announced that it has completed enrollment in the Phase IIb ASCEND study of its novel drug LSTA1 for the treatment of metastatic pancreatic ductal adenocarcinoma (“mPDAC”), achieving a key milestone.
- Italian company Nerviano Medical Sciences (NMS) and Spanish pharmaceutical company Italfarmaco (ITF) jointly announced that ITF will use NMS’s proprietary linker-payload technology to develop a new PDC product candidate.
- Apellis presented post-hoc data for its bicyclic peptide drug EMPAVELI® (pegcetacoplan) at the ASH Annual Meeting that strengthen the efficacy of EMPAVELI (pegcetacoplan) in adults with paroxysmal nocturnal hemoglobinuria (PNH) for up to three years. years of long-term efficacy and safety.
November 2023
- Eli Lilly announced that its GIP/GLP-1 receptor dual agonist Zepbound (Tirzepatide) has been approved by the U.S. FDA to reduce weight and maintain weight stability in obese or overweight adult patients.
- Novo Nordisk released its results for Q3 and the first three quarters of 2023. Sales of semaglutide in the first three quarters have exceeded US$14.2 billion.
- Eli Lilly and Company released its financial report for the third quarter of 2023. Sales of Tirzepatide in the first three quarters have reached US$2.957 billion.
October 2023
- Novo Nordisk announced that the Phase III clinical study FLOW of semaglutide in patients with type 2 diabetes and chronic kidney disease with renal insufficiency was terminated early due to excellent efficacy.
August 2023
- Eli Lilly’s Tirzepatide Injection Submits Registration Application in China for Long-term Weight Management Indication in Adults
July 2023
- Eli Lilly announced the results of two phase 3 clinical trials on tilpotide. The results showed that the average weight loss effect was as high as 26.6%, making it the most powerful weight loss drug in history.
Peptide drugs approved for marketing
December 2023
- Tonghua Dongbao Pharmaceutical’s marketing application for biosimilar GLP-1 analog liraglutide injection filed under registration classification 3.3 has been approved
- Ascendis Pharma announced that the FDA has accepted the company’s resubmitted New Drug Application for palopegteriparatide (TransCon PTH) for the treatment of adult patients with hypoparathyroidism.
- The National Medical Insurance Administration officially announced the adjustment list of the 2023 version of the National Medical Insurance Drug Catalog. The new round of medical insurance catalog includes four popular GLP-1 products. These include Renhui Biotech’s benaglutide injection, Lilly’s dulaglutide injection, Hanson Pharmaceuticals’ polyethylene glycol loxenatide injection, Novo Nordisk’s semaglutide injection, etc.
September 2023
- BioLineRx announces FDA approval of Aphexda (motixafortide) combined with filgrastim (granulocyte colony-stimulating factor G-CSF) to mobilize hematopoietic stem cells into the peripheral blood as a stem cell mobilization (SCM) to promote autologous transplantation in patients with multiple myeloma at the time of transplantation
- The marketing application for new indications of Lilly’s dulaglutide injection (trade name: Duida) was approved
July 2023
- Renhui Biotech’s benaglutide has been approved for marketing as a new indication for weight loss. This is the first original new weight loss drug in China, and the injection frequency is three times a day.
- East China Medicine’s Liraglutide Injection is approved for marketing for obesity or overweight indications
June 2023
- Hansoh Pharmaceutical’s Class 1 new drug “Pemoxatide Injection” (trade name: Saint Relais) was approved for marketing. This medicine is suitable for adult non-dialysis patients who are not receiving erythropoiesis stimulating agents (ESA), and adult dialysis patients who are receiving short-acting erythropoietin (EPO).
May 2023
- Blue Earth Diagnostics’ radiohybrid prostate-specific membrane antigen (PSMA)-targeted PET imaging agent Posluma (flotufolastat F 18) injection (formerly known as 18F-rhPSMA-7.3) was approved by the FDA for marketing
March 2023
- Acadia Pharmaceuticals Announces FDA Approval of DAYBUE (trofinetide) for the Treatment of Rett Syndrome in Adult and Pediatric Patients Two Years and Older
- Cidara Therapeutics’ New Drug Application (NDA) for Rezafungin for the treatment of candidemia and invasive candidiasis was approved by the FDA
January 2023
- Radius Health’s TYMLOS (abapatide) received U.S. FDA approval for new indication (osteoporosis)
Website: https://www.ks-vpeptide.com/ -
Major Advancements in Asymmetric Radical Acylation Unveiled by Anhui Provincial Peptide Drug Lab and Nanjing University in Nature
The Anhui Provincial Peptide Drug Engineering Laboratory (University of Science and Technology of China), in collaboration with a team from Nanjing University, has reported the latest advances in the field of asymmetric radical acylation achieved through photoenzyme catalysis in the journal “Nature.”
In recent times, the team led by Professor Tian Changlin from the Anhui Provincial Peptide Drug Engineering Laboratory (Biomedical Department, University of Science and Technology of China, and the High Magnetic Field Science Center, Chinese Academy of Sciences) collaborated with Professor Huang Xiaoqiang’s team and Professor Liang Yong’s team from Nanjing University to make significant strides in the field of photoenzyme catalysis.
In response to the developed dual catalytic system involving thiamine diphosphate (ThDP)-dependent enzymes and photocatalysis using phosphorus-amino acid (ThDP) as the catalyst, various reaction intermediates, such as free radicals in many reaction processes, changes in the oxidation state of metal catalysts involved in the catalytic reaction, and electron transfer processes during oxidation-reduction, were identified and analyzed using electron paramagnetic resonance (EPR) methods. Professor Tian Changlin’s team at the School of Life Sciences, University of Science and Technology of China, has long been engaged in research at the High Magnetic Field Science Center of the Chinese Academy of Sciences, focusing on the identification of free radicals and analysis of electron transfer in research related to high-field EPR equipment setup, low-temperature EPR method development, and the mechanisms of chemical catalysis and enzyme catalysis, achieving a series of research results (Nat Catalysis 2023; Angew Chem Int Ed 2023, PNAS, 2023, 2022; ACS Catalysis 2023, 2021; Chem Commun, 2022, 2021; Science 2018, etc.). Recently, Professor Tian Changlin’s team collaborated with Professor Huang Xiaoqiang’s team and Professor Liang Yong’s team at Nanjing University to make significant progress in the field of photoenzyme catalysis. Using EPR methods, they identified the free radical intermediates in the newly developed catalytic system and the electron transfer mechanism in the catalytic reaction. The research results, titled “A light-driven enzymatic enantioselective radical acylation,” were published in Nature (DOI: 10.1038/s41586-023-06822-x).
Biomanufacturing is one of the most promising green technologies for transforming industrial sustainability and is a core aspect of enzyme catalysis in synthetic biology. The combination of enzyme catalysis and photocatalysis, known as photoenzyme catalysis, integrates the diverse reactivity of photochemistry with the high selectivity of enzymes, making it the forefront strategy for developing new enzyme functions. The collaborative research team, using a combination of biomimetic and chemical simulation approaches (Figure 1), harnessed visible light excitation and directed evolution to extend enzyme catalytic functions to radical-radical cross-coupling. Additionally, by using directed evolution to modify ThDP-dependent enzymes, they reshaped ThDP-dependent benzaldehyde lyase into a radical acyl transferase (RAT), achieving a non-natural high enantioselective radical-radical coupling reaction.
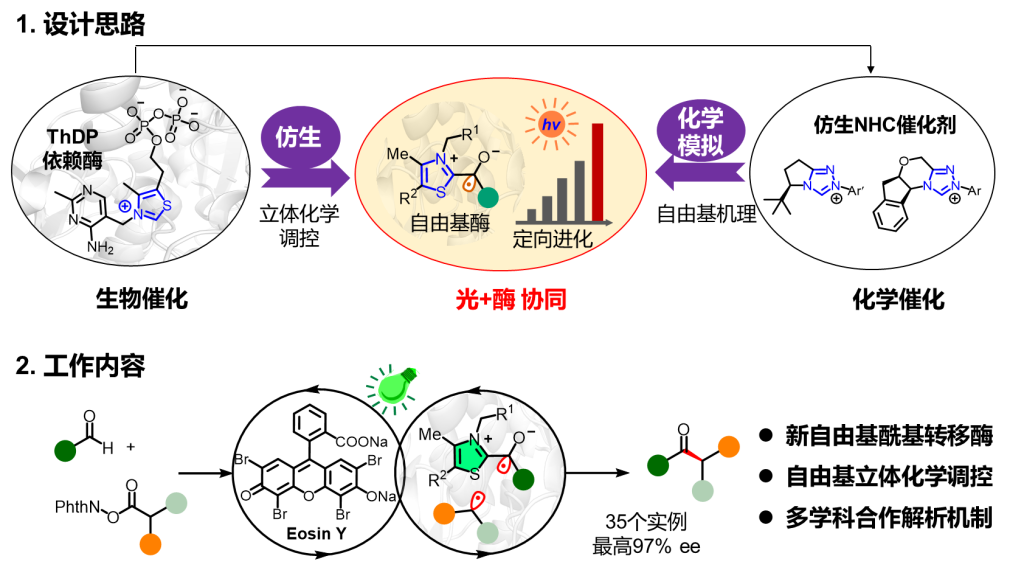
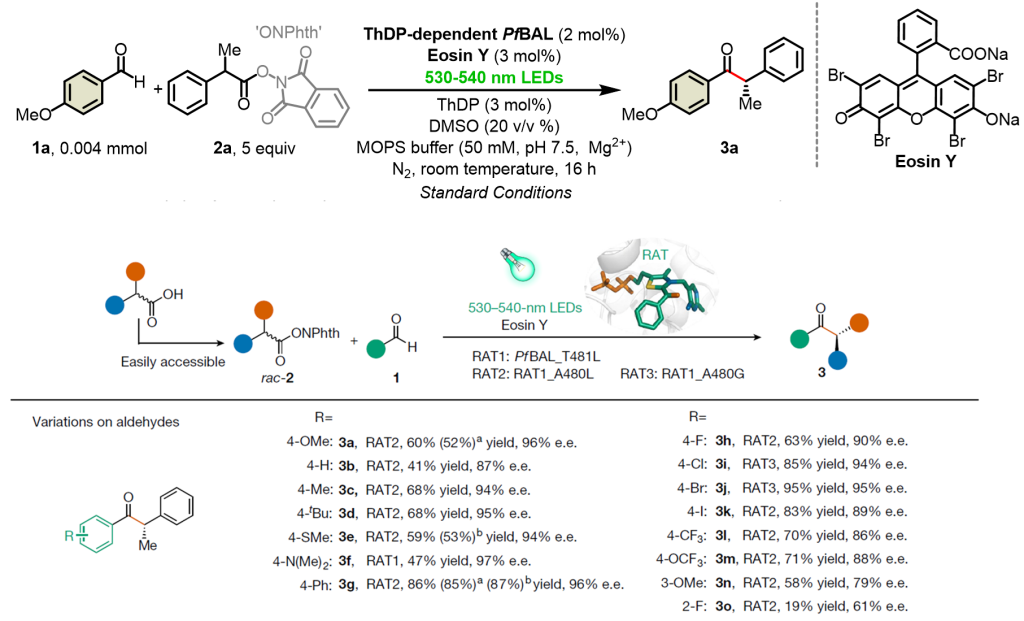
The collaborative team explored the catalytic system of organic dye Rose Bengal and ThDP-dependent enzyme using 4-methoxybenzaldehyde 1a and free radical precursor oxidation-reduction active ester 2a as template substrates. Subsequently, a small and refined mutant library was constructed through molecular dynamics simulations and semi-rational design. The optimal mutant enzyme with high substrate tolerance and substrate selectivity (enantioselectivity up to 97% ee) was obtained, highlighting the finely tuned role of the enzyme’s adjustable active pocket in the stereochemical control of free radical stereochemistry (Figure 2).
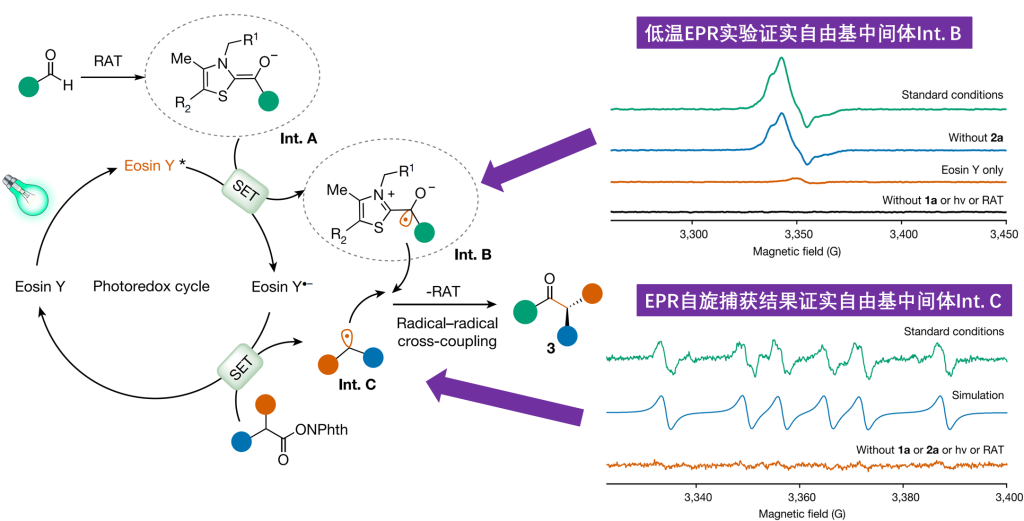
For the photoenzyme dual catalytic system, Professor Tian Changlin’s team applied low-temperature (80K) electron paramagnetic resonance (EPR) experiments, capturing the ThDP-derived ketyl free radical (Int. B). Through EPR spin trapping experiments, they detected characteristic six-line splitting spectra in the standard reaction system, confirming it as an intermediate benzylic radical (Int. C) and the free radical product after addition with the capture agent. This provided direct evidence for unraveling the key to the new enzyme reactivity and the source of high stereochemical selectivity.
The collaborative development of a dual catalytic system combining ThDP-dependent enzyme catalysis and organic photosensitizer Eosin Y catalysis, led by Nanjing University and involving the team from the University of Science and Technology of China, not only transformed natural benzaldehyde lyase into a light-driven radical acyl transferase but also achieved excellent stereochemical control of a challenging prochiral free radical. Nanjing University is the first and last corresponding author unit, and the University of Science and Technology of China and the Anhui Provincial Peptide Drug Engineering Laboratory are co-corresponding author units. The aforementioned research work received funding from the National Natural Science Foundation of China’s Outstanding Youth Fund, major instrument development projects, and the Ministry of Science and Technology’s key research and development program.
Website: https://www.ks-vpeptide.com/products.html -
Casgevy and Lyfgenia – why they are milestones and why they are just milestones
On December 8th, the U.S. Food and Drug Administration (FDA) approved two groundbreaking therapeutic drugs, Casgevy (developed jointly by Vertex Pharmaceuticals and CRISPR Therapeutics) and Lyfgenia (developed by Blue Bird Bio). These drugs represent the first wave of cell-based gene therapies approved by the FDA for the treatment of sickle cell disease (SCD) in patients aged 12 and above. Notably, Casgevy is the first FDA-approved therapeutic drug utilizing CRISPR gene editing technology, marking a significant milestone in the field of gene therapy.
Indications of Casgevy and Lyfgenia:
- Casgevy:
- Indication: Treatment for patients aged 12 and above with recurrent vaso-occlusive crises (VOCs) in sickle cell anemia (SCD).
- Lyfgenia:
- Indication: Treatment for patients aged 12 and above with a history of vaso-occlusive events (VOEs) in sickle cell disease.
It’s worth mentioning that Lyfgenia’s parent company, Blue Bird Bio, received approval for another drug called Zynteglo in August 2022, intended for the treatment of transfusion-dependent β-thalassemia (TDT).
Pathogenesis and Therapeutic Mechanism:
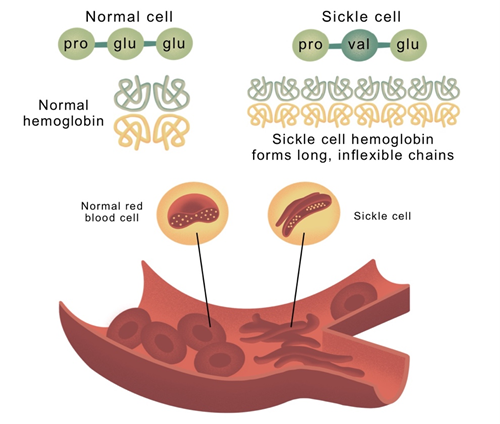
The structural composition of human hemoglobin consists of a heterotetramer, composed of two pairs of globin proteins. SCD and TDT result from variations in the β-globin protein. SCD involves a mutation where Glu6 in β-globin is replaced by Val6, causing hemoglobin to form long chains through hydrophobic interactions, leading to sickle-shaped cells and reduced oxygen-carrying capacity. TDT results from deficient synthesis of the β-globin chain, causing an excess of unbound α-chains, leading to precipitation and hemolysis within red blood cells.
- Casgevy’s Strategy:
- Utilizes CRISPR technology to knock out the BCL11A gene.
- BCL11A is a transcription factor that suppresses the expression of fetal hemoglobin (HbF).
- Knocking out BCL11A results in upregulation of γ-globin expression and downregulation of β-globin, leading to the production of functional fetal hemoglobin (HbF).
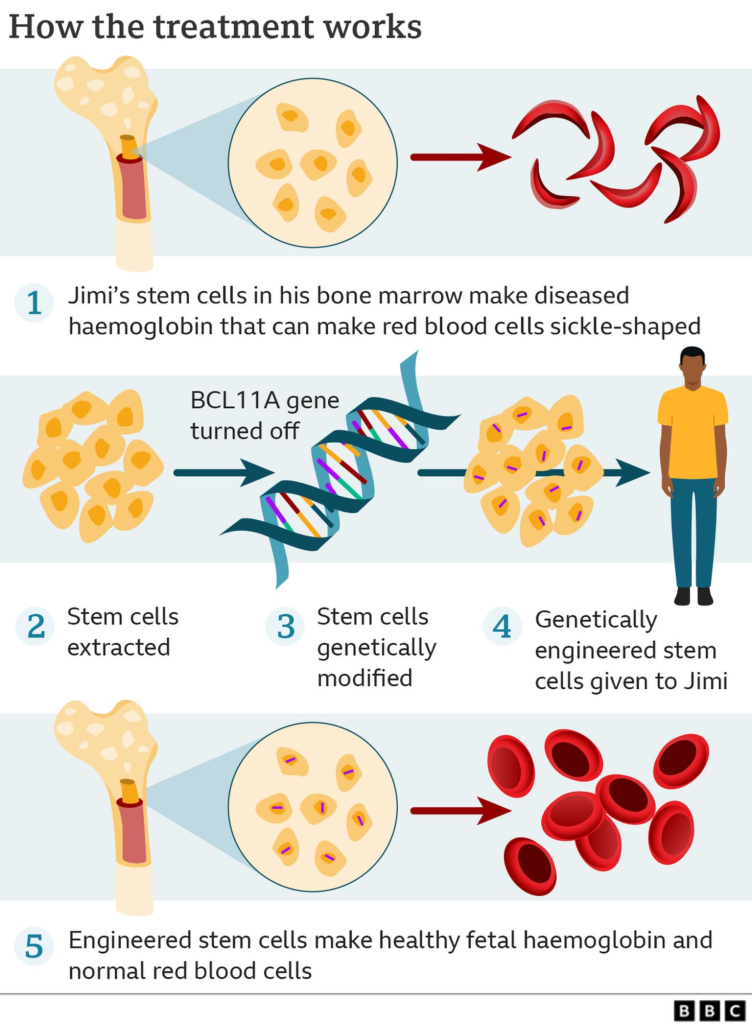
- Lyfgenia’s Strategy:
- Uses a lentivirus to insert an enhanced β-globin (T87Q) into the patient’s hematopoietic stem cells.
- Generates hemoglobin (HbAT87Q) with anti-sickling properties, reducing the occurrence of VOEs.
- HbAT87Q exhibits oxygen-carrying capacity similar to wild-type hemoglobin.
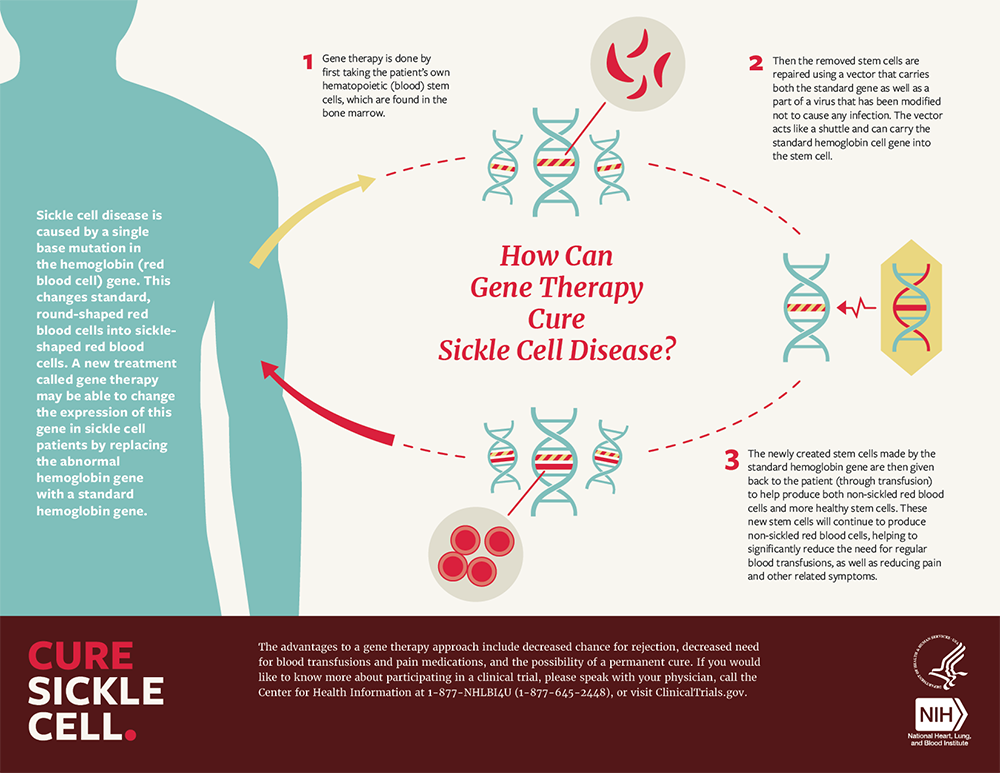
Both treatments involve extracting bone marrow hematopoietic stem cells from the patient, editing them in the laboratory using either CRISPR or viral vectors, and then reintroducing the edited stem cells into the patient.
Why (Only) Milestones:
While Casgevy and Lyfgenia represent significant milestones in the treatment of SCD, certain factors limit them from being the ultimate solutions. Safety concerns, potential carcinogenic risks associated with viral vectors (as in the case of Lyfgenia), and the long-term safety of gene editing are issues that need further investigation. Both therapies also involve myeloablation (bone marrow conditioning), leading to immune and reproductive challenges.
Another critical factor is the pricing. With Casgevy priced at $2.2 million and Lyfgenia at $3.1 million, the high costs raise questions about accessibility and fairness, especially for SCD patients in sub-Saharan Africa. Traditional treatments, while not curative, are significantly more affordable.
In conclusion, Casgevy and Lyfgenia are indeed milestones, but they are not the endpoint for SCD. The emergence of gene editing therapies marks the beginning of a new era, providing hope for revolutionary treatments. However, challenges such as safety concerns, accessibility, and pricing highlight the need for continuous advancements and ethical considerations in the evolving landscape of gene therapies. As we witness these groundbreaking treatments, Winston Churchill’s words come to mind: “Now this is not the end. It is not even the beginning of the end. But it is, perhaps, the end of the beginning.” The advent of these therapies signifies a new chapter, and while celebrating their potential, we must also address the challenges and questions they bring.
If you want to know more about drug discovery, please visit https://www.ks-vpeptide.com
- Casgevy:
